Improving Early Detection of Bladder Cancer
The Problem with the Current Approach
Bladder cancer is the sixth most common cancer in the United States—and one of the deadliest when diagnosed late. When found early, it is highly treatable. So why are so many cases missed?
-
Low referral rates – Many patients with blood in the urine (hematuria) are never referred to a urologist, especially women, who are more likely to be misdiagnosed or experience delays.
-
Delayed diagnosis – Even among those referred, the path to diagnosis can take months or longer.
-
Missed cancers – Traditional tools like urine cytology and imaging can miss early-stage tumors, particularly small or flat lesions.
-
High mortality – Once bladder cancer spreads, survival rates decline sharply.
Despite clear clinical guidelines, the system remains inconsistent—and too many patients fall through the cracks.


A Better Path with AssureMDx
AssureMDx is a simple, non-invasive urine test that detects genomic changes associated with the presence of bladder cancer. It helps identify patients who should be referred to a urologist sooner—and offers reassurance for those at lower risk.
What makes AssureMDx different:
-
Uses a standard urine sample—no special collection process or preparation
-
Can be ordered by any clinician, including primary care or gynecology
-
Helps detect cancer earlier, when treatment is most effective
-
Reduces unnecessary procedures in patients unlikely to have cancer
-
Supports more informed, risk-based referrals to urology
AssureMDx gives patients and providers better information, earlier—helping close the gaps in bladder cancer diagnosis and improving confidence in clinical decisions.
AssureMDx Improves Risk Stratification and Early Detection
AssureMDx isdesigned to assist in the risk stratification of patients presenting with hematuria. By analyzing key DNA methylation and mutation biomarkers, AssureMDx helps identify patients at risk for urothelial carcinoma (UC), enhancing clinical decision-making and enabling earlier, more targeted urologic evaluation.
-
Intended Use: Evaluation of patients with gross or microscopic hematuria to support triage for urologic evaluation.
-
Methodology: Methylation-specific PCR and next-generation sequencing to assess DNA methylation (TWIST1, OTX1, ONECUT2) and somatic mutations (FGFR3, HRAS, TERT).
-
Sample Type: Urinary exfoliated cell DNA from a voided urine sample.


Backed by Evidence. Built for Better Decisions.
Vesica's biomarkers and technology are supported by over 26 peer-reviewed publications and data from more than 8,500 patients, including three clinical validation studies on AssureMDx involving 1,192 patients presenting with hematuria.
The AssureMDx test consistently delivers high sensitivity, specificity, and negative predictive value, with AUCs up to 0.97. (1-4)
A positive test result identifies patients at increased risk—including those who may be missed by cystoscopy alone—helping guide earlier, more intensive follow-up when it matters most.
A negative test result provides strong reassurance and helps avoid unnecessary procedures, thanks to AssureMDx’s >99% negative predictive value. This supports more confident, efficient care for patients truly at low risk.
References
1) van Kessel et al, J Uro 2016, 2) van Kessel et al J Uro 2017, 3) van Kessel et al, 2020 4) de Jong et al, Eur Uro Onc 2023
A Urine-based Genomic Assay Improves Risk Stratification for Patients with High-risk Hematuria Stratified According to the American Urological Association Guidelines
de Jong, Joep J. et al., European Urology Oncology 2023
Study Summary: This peer-reviewed analysis examined how AssureMDx complements AUA Hematuria Risk Stratification guidelines using a prospective cohort of 838 patients. The study used the same population as van Kessel et al. (2020) but focused on applying guideline-defined risk categories (low, intermediate, and high) to evaluate whether the assay could enhance risk-based decision-making. The test detects genomic alterations in urinary DNA, including methylation of TWIST1, OTX1, ONECUT2 and somatic mutations in the FGFR3, TERT, HRAS genes.
Best-in-Class Performance:
-
AUC of 0.96
-
Overall sensitivity 96%
-
Negative Predictive Value (NPV) >99%
Key Takeaway: AssureMDx detects urothelial carcinoma with high sensitivity across both early and advanced stages. Notably, AssureMDx achieved a combined sensitivity of 95.9% for Stage 0 (Ta and Tis/CIS) and Stage I disease—underpinning its unique ability to identify patients at increased risk for bladder cancer at its earliest and most treatable stages.

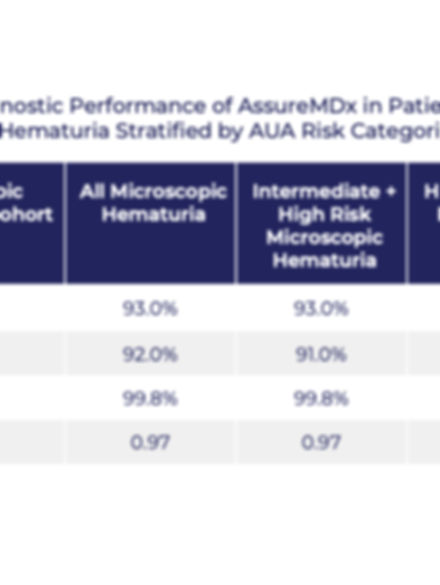
AssureMDx Performance by AUA Micro Hematuria Risk Classification
de Jong, Joep J. et al., European Urology Oncology 2023
This table presents the sensitivity, specificity, AUC, and negative predictive value (NPV) for AssureMDx in patients with microscopic hematuria, further stratified by intermediate and high-risk categories based on the 2020 AUA Hematuria Guidelines. The assay achieved AUC values of 0.97 and NPV of 99.8% across all groups—supporting its value as a non-invasive triage tool in a lower-prevalence population.
Why This Matters
Gross Hematuria: In patients with visible blood in the urine—where cancer risk is higher—AssureMDx helps detect cases that cystoscopy may miss, including flat or early-stage lesions. A positive result supports more intensive follow-up; a negative result offers added reassurance with >99% NPV.
Microscopic Hematuria: In lower-risk patients with non-visible hematuria, AssureMDx combines high sensitivity and specificity to better pinpoint who truly needs further urologic evaluation—enabling earlier detection for some and safely avoiding procedures for others.
A Urine Based Genomic Assay to Triage Patients with Hematuria for Cystoscopy.
van Kessel KEM, de Jong JJ, Ziel-van der Made ACJ, et al., Journal of Urology 2020
Study Summary: This prospective, multicenter study enrolled 838 hematuria patients with either gross or microscopic hematuria across six centers. Urine samples were collected prior to cystoscopy and analyzed using the validated DNA-based assay targeting six genomic biomarkers: methylation of TWIST1, OTX1, ONECUT2, and mutations in FGFR3, TERT, and HRAS.
AssureMDx achieved an AUC of 0.95, with 96% sensitivity and a negative predictive value of 99.7%, demonstrating strong diagnostic performance and supporting its use as a non-invasive triage tool to reduce unnecessary cystoscopies while improving early cancer detection.
Figure 3. ROC Curve – All Hematuria Patients (n=838) The ROC curve illustrates the diagnostic accuracy of AssureMDx across the entire hematuria cohort, including both gross and microscopic presentations. The model achieved an AUC of 0.957, demonstrating robust overall discriminative ability for urothelial carcinoma detection.
Figure 4. ROC Curve – Microscopic Hematuria Subgroup (n=381) In this lower-prevalence, clinically challenging population, AssureMDx maintained exceptional diagnostic accuracy, achieving an AUC of 0.971. This supports its utility as a highly effective triage tool even in cases where disease may be more difficult to detect.
Why this Matters: This study demonstrated that AssureMDx delivers consistently high sensitivity and negative predictive value across the full spectrum of urothelial cancer—from early, low-grade tumors to advanced, high-grade disease. These findings support its use as a reliable triage tool for patients with gross or microscopic hematuria, helping ensure timely cancer detection while reducing unnecessary procedures.

Validation of a DNA Methylation-Mutation Urine Assay to Select Patients with Hematuria for Cystoscopy.
van Kessel KE, Beukers W, Lurkin I, et al., Journal of Urology 2017
Study Summary: This was a prospective, multicenter clinical validation study involving 200 patients with hematuria. The assay assess the DNA methylation status of OTX1, ONECUT2, TWIST1 markers with somatic mutations in FGFR3, TERT, and HRAS genes, collected and analyzed prior to cystoscopy.
-
Overall AUC: 0.96 (95% CI 0.92–0.99)
-
Sensitivity: 93%
-
Specificity: 86%
-
Negative Predictive Value (NPV): 99.6%
Key Takeaway: AssureMDx demonstrated high sensitivity for both low- and high-grade tumors, strong overall discriminative power, and value as a non-invasive triage tool for hematuria patients.
Evaluation of an Epigenetic Profile for the Detection of Bladder Cancer in Patients with Hematuria.
van Kessel KE, Van Neste L, Lurkin I, Zwarthoff EC, Van Criekinge W. et al., Journal of Urology 2016
Study Summary: This was a retrospective, case-enriched validation study (n=154) evaluating the six-biomarker urine assay composed of DNA methylation markers OTX1, ONECUT2, and TWIST1 and DNA mutations in the FGFR3, TERT, and HRAS genes. The study included 74 bladder cancer cases and 80 controls.
-
Overall AUC: 0.93 (95% CI 0.88–0.98)
-
Sensitivity: 97%
-
Specificity: 83%
-
NPV: ~99.8% (based on assumed prevalence)
Key Takeaway: This study provided initial validation of the full biomarker panel, demonstrating excellent performance in distinguishing bladder cancer cases from controls. It set the foundation for the prospective, multicenter validation conducted in the 2017 study.
Get in Touch
Vesica Health, Inc.
5 Mason Lane
Suite #180
Irvine, California 92618
California State Department of Health Clinical Laboratory License: CDF-90001843
CLIA License: 05D2197032
CAP Accreditation: 9647012
For more information about Vesica Health and AssureMDx, please complete the following:

